PIPELINE
All contents and Orders moved to new Hompage (www.nanoglia.com)
- Drug or any gene regulators, including siRNA, plasmid DNA, AAV vector or CRISPR can be loaded in PLGA nanoparticles. Encapsulation efficiency of PLGA NPs is about 30% which is proved by drug releasing assay.
Introduction
Amidst
the various polymers synthesized for formulating polymeric nanoparticles, poly(lactic-co-glycolic
acid) (PLGA) is the most popular. PLGA has
several interesting properties such as controlled and sustained release, low
cytotoxicity, long-standing biomedical
applications, biocompatibility with tissues and cells, prolonged residence time
and targeted delivery.
PLGA has
already been approved by the US FDA as a therapeutic device owing to its
biodegradability and biocompatibility. PLGA NPs are
one of the potential device to deliver a various regulators, including siRNA,
plasmid DNA, AAV vectors or CRISPR, which regulate the gene expression.
Product Specifications
-
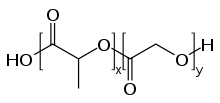
Formula :
- Storage/Stability :
1. -20 ℃
with powder for long time
2. Solubility :Soluble in autoclaved water or PBS stable at +4℃ for 1-2 weeks.